Usp Purified Water Conductivity

Note that you cannot fail.
Usp purified water conductivity. E g as described in iso 7888 water quality determination of electrical conductivity. Purified water packaged in bulk for commercial use elsewhere meets the requirements of all of the tests under sterile purified water except labeling and sterility 71. You may purchase usp24 by calling customer service at 800 877 6733. A balancing quantity of cations such as sodium ion is.
The usp is also available at pharmacy colleges. Usp24 contains complete versions of all pharmaceutical water monographs p. 1927 1929 and 1231 water for pharmaceutical purposes p. Purified water is also referenced throughout the usp nf.
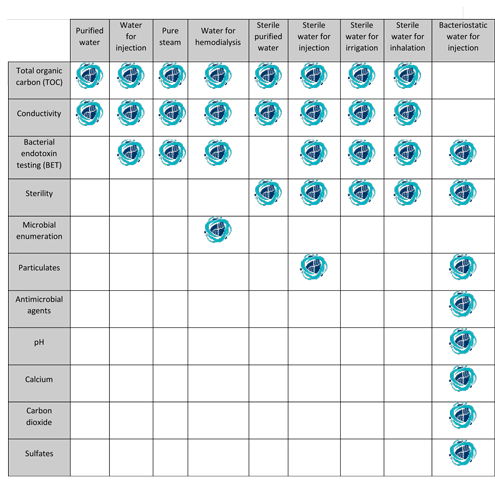
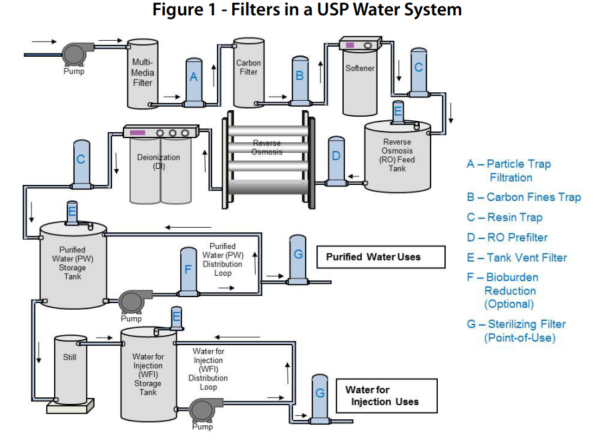
The tests for total organic carbon and conductivity apply to purified water produced on site for use as an ingredient of official preparations and in tests and assays. Yes this is correct. The procedure described in the section bulk water is designed for measuring the conductivity of waters such as purified water water for injection water for hemodialysis and the condensate of pure steam produced in bulk. For water packaged in bulk but manufactured elsewhere or for sterile purified water sterile water for injection sterile.
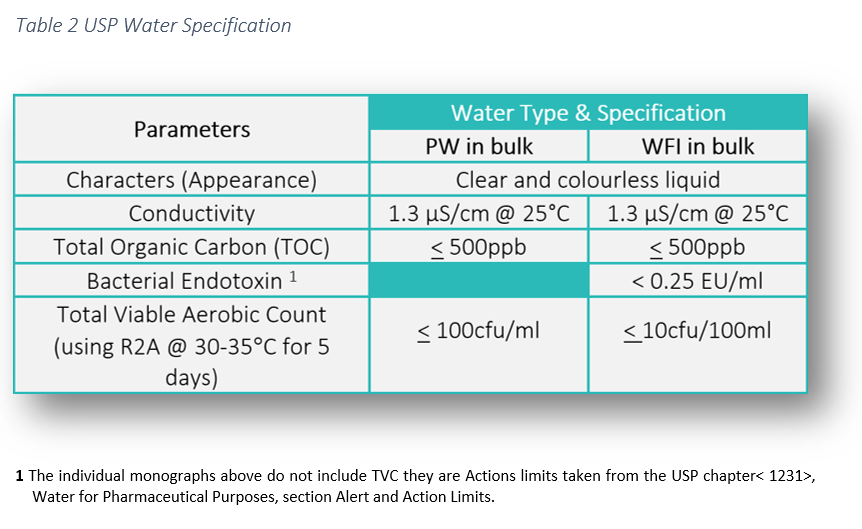
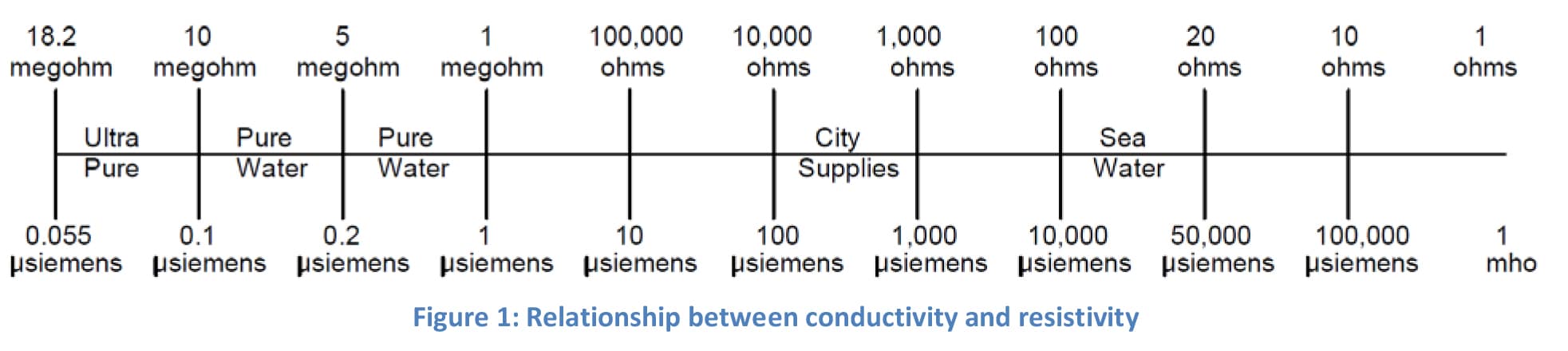
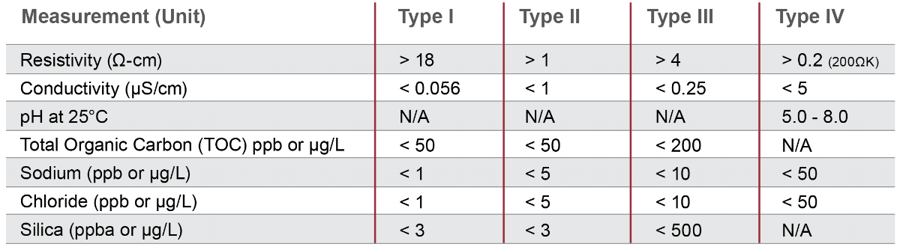
The heavy metals test on usp waters was deleted in 1996. Conductivity measurements are a useful indicator of the amount of dissolved ions present in a water sample and can serve as a measure of water quality. Water conductivity is used as a measure of purity for bulk usp purified water pw and water for injection wfi in the pharmaceutical industry. Unless otherwise specified purified water is also to be used for all tests and assays for which water is indicated see general notices and requirements.
1752 1754 and the general chapters 643 toc 645 water conductivity p. The conductivity of the ubiquitous chloride ion at the theoretical endpoint concentration of 0 47 ppm when it was a required attribute test in usp xxii and earlier revisions and the ammonium ion at the limit of 0 3 ppm represents a major portion of the allowed water impurity level. The usp purified water and the usp wfi on the other hand are components or ingredient materials as they are termed by the usp intended to be used in the production of drug products. Regardless of the font and letter case used in its spelling water complying with the purified water monograph is intended.
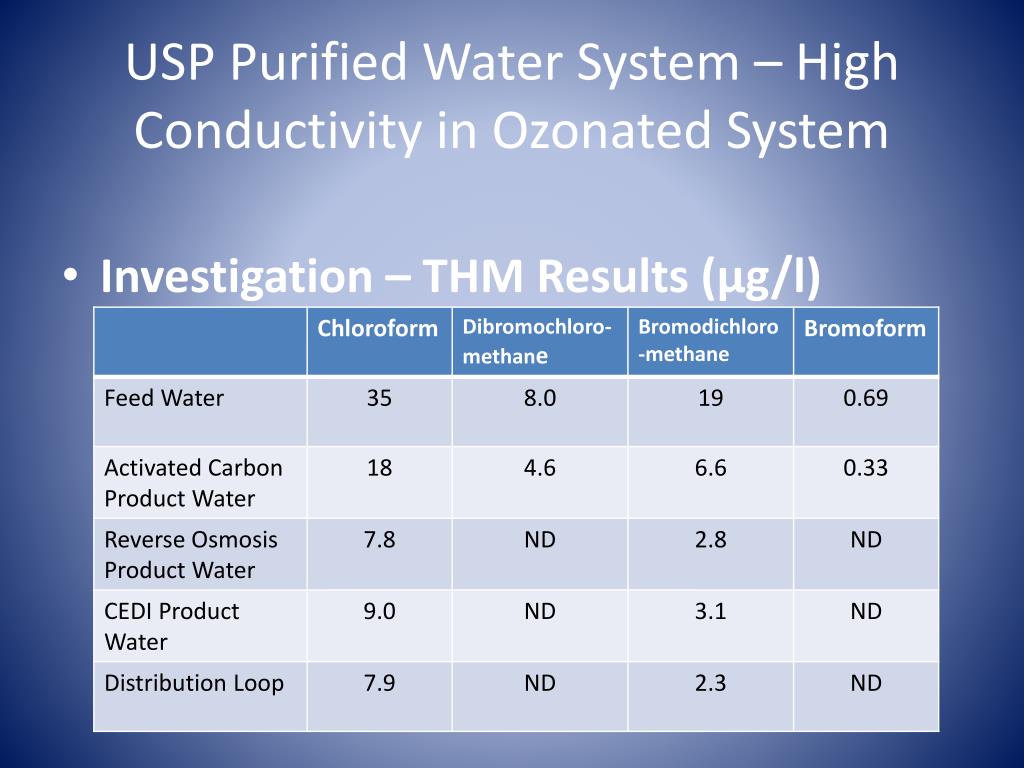
We have established a process purified water charcoal treatment softening uv sanitization and 0 2um filtration while the water quality is better than drinking water but not good enough to fulfill usp ep water specification only conductivity higher than the criteria 2 3us cm others are all within spec. The ph test was deleted in 1998. Conductivity testing is more reliable and. There has never been a test for nitrates for usp waters.
Including total vial count and. One is for the intrinsic conductivity of water and the other is for the other ionic species in water.