Xcela Power Injectable Port Problems

300 psi 19 gauge and 20 gauge needles with 5 ml s maximum infusion rate.
Xcela power injectable port problems. Indicated for power injection of contrast media up to 5 ml sec. The complaint stated that 2 xcela ports were returned because the catheter was fractured up near the port. This product does not contain dehp. Identification of the powerport device under x ray.
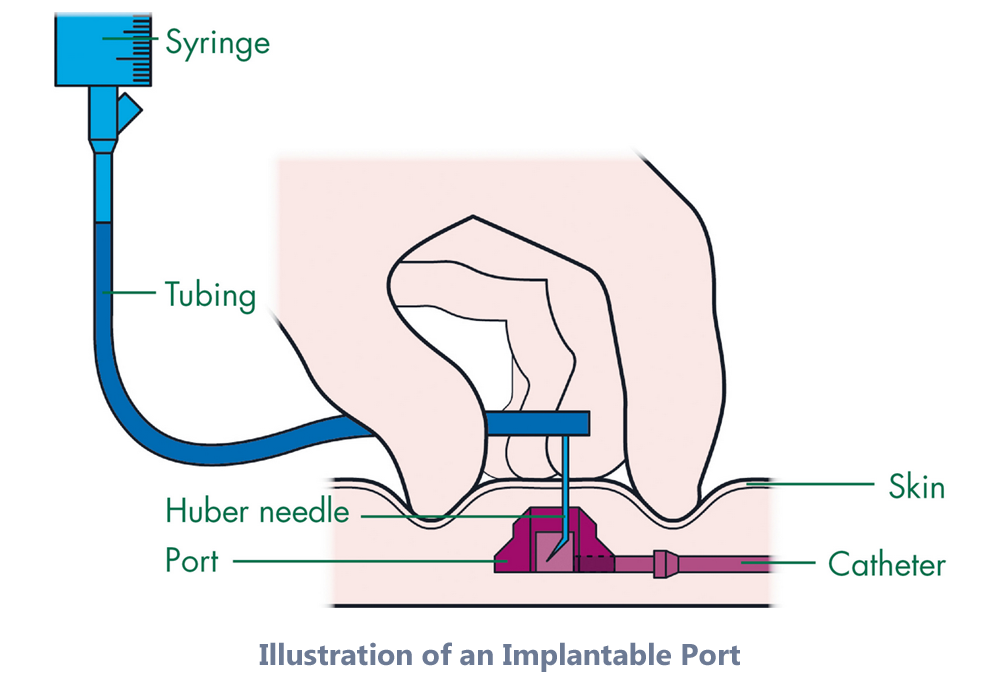
A device history record review was conducted for the reported lots. Easily identify critical information. Designed for patient comfort. Most implanted ports are made to be used during imaging tests such as computed tomography ct scans or magnetic resonance imaging mri to allow for high speed injections shots of contrast.
Accessgudid xcela h965451090 6 6f x 750mm xcela low profile titanium power injectable port with silicone plug. Smart port ct injectable port. Vascular access vortex ports. The product in question was not returned to b 6 medical for evaluation.
Accessgudid xcela h965451810 9 6f x 500mm xcela plastic dual lumen power injectable port with attachable polyurethane catheter. Xcela plus ports with pasv valve technology. Power injection and cect scan capable when used with powerloc safety infusion set. The records are complete and in order.
The smart port ct family of power injectable ports which include low profile and mini models. These implanted ports are called. Reference b 4 mdr 203582 2012 00015 for other port included in complaint. The power of pasv.
Your port may be called a bardport a mediport a powerport or a port a cath. Note that the word ct is visible on a x ray image of the newer models of ports as an identifier that this port is power injectable. Lower insertion and penetration force than non siliconized port access needles. The pasv valve technology design automatically resists backflow reducing blood reflux that could lead to catheter related complications.
300 psi 22 gauge needle with 2 ml s maximum infusion rate. Radiopaque ct lettering confirms if port is power injectable or flipped. Radiopaque identifier on the bottom of the port to aid in. A problem was reported to b 6 medical regarding the xcela power injectable port list h965451090 lot no s 1027cs 1030cs 1008cs.
The powerport by bard is a subcutaneous indwelling central venous access port that is fda approved for power injection of contrast. An investigation of the returned product indicated that only one of the two ports returned had a fracture of the catheter.